OPIOID DEPENDENCY
DETOX
Opioid dependency can cast a shadow over your life, but the path to reclaiming your independence shines bright with FDA-cleared S.T. Genesis.
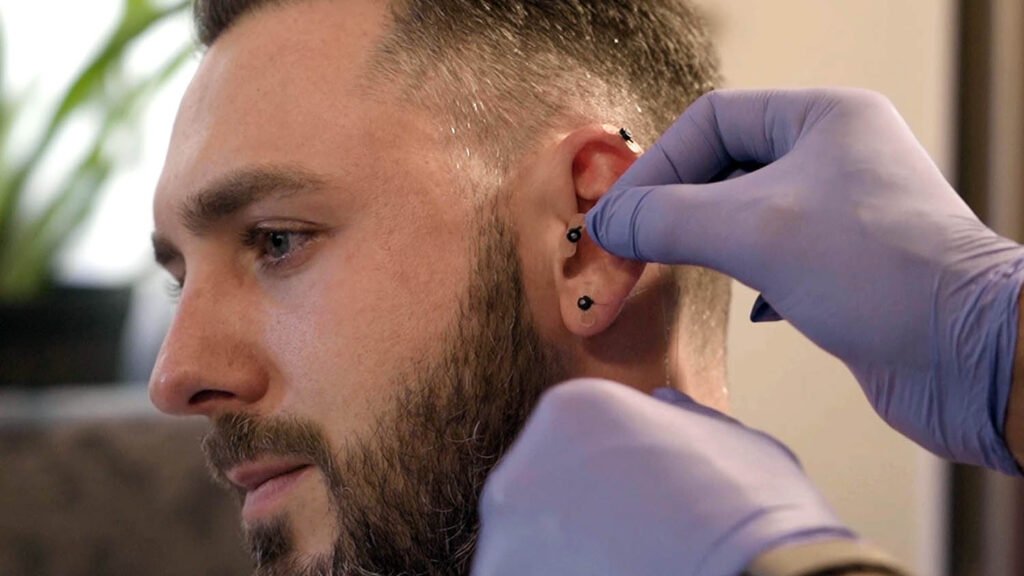
Break free from the cycle of opioid dependency with S.T. Genesis, the FDA-Cleared Percutaneous Nerve Field Stimulator (PNFS).
S.T. Genesis (PNFS)
S.T. Genesis is an FDA-cleared Percutaneous Nerve Field Stimulator (PNFS) that supports a patient’s reduction of opioid withdrawal symptoms. Stimulation is performed by sending electrical pulses emitted through needles strategically positioned in the ear to branches of cranial nerves V, VII, IX, and X as well as the occipital nerves.
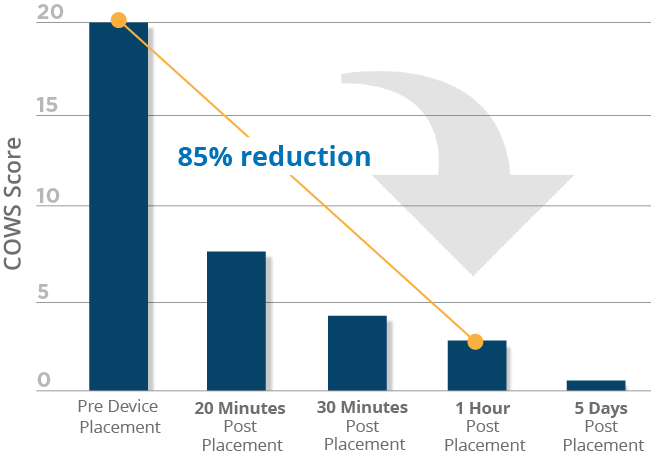
Clinical data shows that nearly all adult patients included in the study successfully transitioned from the use of the device during detoxification (medically supervised opioid withdrawal) to medication assisted therapy (MAT). A reduction in Clinical Opioid Withdrawal Scale (COWS) scores was noticeable within 20 minutes of device placement, with scores continuing to drop over the five-day duration of opioid withdrawal treatment. This effective approach has been shown to allow patients to progress with recovery with the support of physician follow-up along with MAT.*
*Adrian Miranda & Arturo Taca (2018), The American Journal of Drug and Alcohol Abuse, 44:1, 56-63.
FAQs
-
Patients with visible withdrawal symptoms (such as sweating, increased pulse rate, dilated pupils, nausea, vomiting) and those who measure with mild to severe withdrawal symptoms on the Clinical Opiate Withdrawal Scale (COWS) score.
-
S.T. Genesis is an FDA-cleared Percutaneous Nerve Field Stimulator (PNFS) that supports a patient’s reduction of opioid withdrawal symptoms. Stimulation is performed by sending electrical pulses emitted through needles strategically positioned in the ear to branches of cranial nerves V, VII, IX, and X as well as the occipital nerves.
-
Speranza, the manufacturer of S.T. Genesis Treatment understands the difficulties in navigating the pathways to insurance reimbursement within the neuromodulation industry. That’s why they have created a separate division called Reimbursement Support Services. This division has been built with the patient and provider in mind to create seamless options to reimbursement and to ease the burden of obtaining proper reimbursement for the neuromodulation patient.
For assistance with the program forms or referral submission, please contact Speranza Support at 1-866-923-0710.
S.T. Genesis: The First Step in the OUD Patient’s Continuum of Care
As the opioid use disorder (OUD) patient begins the journey to recovery, creating the best and most effective continuum of care can be a matter of life or death. Relapse rates in OUD patients post-detoxification are higher than any other drug, with 88% relapsing after 12 to 36 months.
READ MORE
EDUCATION & ARTICLES
EDUCATION & ARTICLES
A New Wave in the Opioid Epidemic – The Rise of Fentanyl Laced Drugs
In recent years, there has been an extreme rise in illicitly produced fentanyl being mixed into other drugs such as cocaine, counterfeit prescription pills, and methamphetamines. This, coupled with the increase in drug use and isolation from the pandemic, has...
Neuromodulation: Emerging Innovation and Greater Adoption
Neuromodulation works by actively stimulating nerves to produce a natural biological response. It is the alteration—or modulation—of nerve activity by delivering electrical stimulation directly to a targeted area of the central nervous system.1 Neuromodulation devices may be invasive or noninvasive.